The conclusions and ultimate assessments has to be comprehensively documented and reviewed with the Certified Individual ahead of the product or service batch is accredited to be used.
Each and every manufacturing process phase is managed in order that the completed product or service satisfies all described quality characteristics.
The purpose of process validation is to make sure that the Management tactic is sufficient for the process layout and item high quality. The validation process should incorporate all strengths of the merchandise along with the output internet sites useful for manufacturing the products.
By validating a process, companies can decrease the threat of producing faulty items, lessen the incidence of deviations, and prevent highly-priced recalls.
Hazard evaluation plays an important part in process validation. By pinpointing prospective threats and areas of problem, organizations can emphasis their validation initiatives on essential process parameters and steps.
4. Revalidation: When you will discover modifications in packaging, formulation, equipment or processes which could have effect on merchandise success or item attributes, there needs to be revalidation from the validated process.
Documented proof performs a crucial part while in the FDA's process validation tactic. The pointers emphasize the necessity for complete documentation to demonstrate process Regulate and be certain repeatability and reproducibility.
Process validation studies could possibly be carried out on pilot-scale batches for products not still scaled to total output levels. These pilot batches need to characterize at the least ten% with the production scale batch size, ensuring that the dimensions-up variable isn't going to exceed tenfold.
Danger assessment performs a vital position in process validation. By determining probable challenges and regions of problem, organizations can emphasis their validation attempts on critical process parameters and steps.
This not merely aids to make certain product good quality and client security but will also demonstrates compliance with regulatory expectations.
Process validation is vital for demonstrating compliance with regulatory standards, for example These established by the FDA or ISO. It provides documented proof that processes are managed and able of producing high quality products and solutions, which can be important for passing audits and staying click here away from authorized issues.
Translating the sources of variability right into a properly-developed control tactic, that reliably makes sure an item’s attributes are attained, might assistance obtain strong merchandise realization.
Possibility evaluation and mitigation: Incorporating threat evaluation into your process validation aids identify opportunity troubles prior to they come to be considerable troubles. By analyzing attainable hazards associated with Just about every process stage, you can put into practice methods to mitigate them, making sure smoother functions.
Possessing a very clear roadmap that makes sure every single merchandise you build meets the very best benchmarks of quality and safety. That’s what process validation is centered on. It’s more info a systematic approach that assists you validate that the manufacturing processes regularly develop effects that meet predetermined specs and quality characteristics.
 Angus T. Jones Then & Now!
Angus T. Jones Then & Now!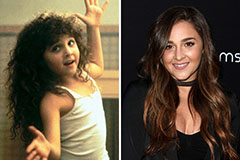 Alisan Porter Then & Now!
Alisan Porter Then & Now! Andrew Keegan Then & Now!
Andrew Keegan Then & Now!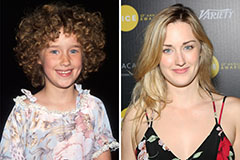 Ashley Johnson Then & Now!
Ashley Johnson Then & Now! Christy Canyon Then & Now!
Christy Canyon Then & Now!